1. Brain iron metabolism dysfunction in Parkinson's disease.
Recently, increasing evidence suggests that iron plays a key role in Parkinson's disease (PD) pathogenesis. In our studies, redox-active iron, as a potent pro-oxidant, participates in the dopaminergic neuronal death in PD [1]. We also discovered that iron accumulated in the substantia nigra (SN) in PD models but decreased in the temporal cortex in postmortem human brains with PD [2-4]. Through binding to iron responsive elements (IREs), iron responsive proteins (IRPs) are able to posttranscriptionally regulate mRNAs that have IREs in the 3′- or 5′- UTRs, control iron uptake via TfR1and DMT1, and export via FPN1 with the help of HP and GPI-CP [5-10]. In addition, the degradation of DMT1 can be regulated by the ubiquitin-proteasome system, such as parkin, Ndfip1 [1, 2, 11].
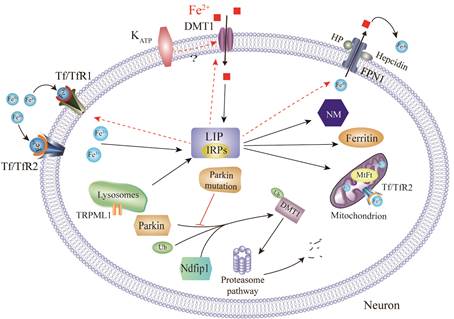
DMT1: Divalent metal transporter; MtFt: Mitochondria ferritin; FPN1: Ferroportin1;
NM: Neuromelanin; HP: Hephaestin; Tf: Transferrin; IRPs: Iron regulatory proteins;
TfR: Transferrin receptor; LIP: Labile iron pool; Ndfip1: Nedd4 family-interacting protein 1;
TRPML1:Transient receptor potential mucolipin 1; Ub: Ubiquitin; KATP: ATP-sensitive potassium channels.
2. Interactions between iron accumulation and α-synuclein aggregation.
Both α-synuclein (α-syn) aggregation and iron deposit are neuropathological hallmarks of PD. The potential link between iron and α-syn has been consistently observed. Our recent work showed that iron could induce α-syn aggregation [12, 13], which is partially dependent on oxidative stress and IRE/IRP system [14]. Moreover, iron and α-syn may act synergistically to induce neurotoxicity by inhibiting Nrf2/ HO-1 pathway, initiating a vicious cycle of iron accumulation, α-syn aggregation and HO-1 disturbance in PD [15].
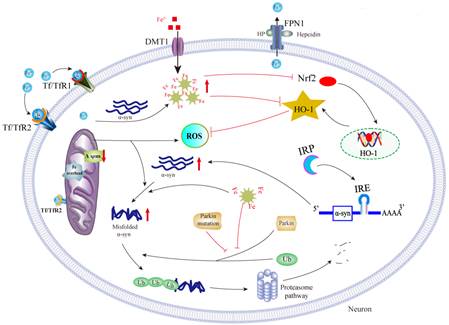
ΔΨm: Mitochondrial transmembrane potential; ROS : Reactive oxygen species; HO-1: Heme oxygenase-1;
IRE : Iron responsive element; Nrf 2: Nuclear factor erythroid-2-related factor 2.
3.
Astrocytes
and microglia contributions to iron metabolism disturbance in PD.
Neurodegenerative processes in PD trigger universal and conserved astrocytes and microglia reactions. Much effort has been devoted to understanding the neuronal changes under the condition of disturbed iron status in PD, however, we are particularly interested in how astrocytes and microglia contribute to iron metabolism disturbance in PD. We reported iron trafficking rate and mitochondrial iron sequestration were different between neurons and astrocytes [16, 17]. BDNF and GDNF derived from astrocytes, and proinflammatory cytokines and lactoferrin derived from microglia, might act on intracellular IRPs and thus modulate neuronal iron accumulation, or exert neuroprotective effects [18-20].
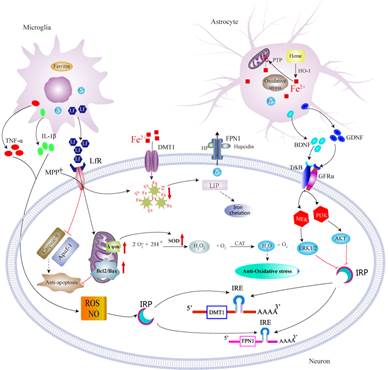
Lf: Lactoferrin; LfR: Lactoferrin receptor; MPP+:1-Methyl-4-phenylpyridinium.
4. Ghrelin might be potentially targeted in PD.
Non-motor symptoms are attracting attentions in PD. Gastrointestinal dysfunctions are particularly early events in PD patients. Hormones operating to regulate gastrointestinal function include ghrelin and nesfatin-1 [21-23]. We demonstrated that ghrelin could increase neuronal excitability by inhibiting voltage-gated potassium Kv7/KCNQ channels through its receptor GHS-R1a and activation of the PLC-PKC pathway [24], and exerted neuroprotective effects on dopaminergic neurons and contributed to hippocampal neurogenesis [25, 26], which suggested that ghrelin might be a potential target as therapeutic strategies in PD.
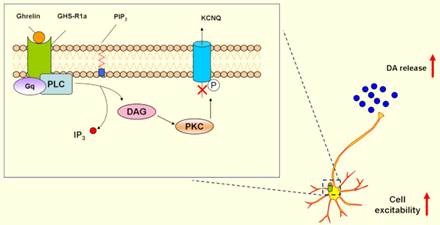
DAG: Diacylglycerol; GHS-R1a:Growth hormone secretagogue receptor 1a;
DA: Dopamine; PLC: Phospholipase C; PKC: Protein kinase C;
IP3: Inositol1,4,5-triphosphate; IP3: Inositol1,4,5-triphosphate.
For more information, please refer to the Published Papers:
1. Jia W#, Xu H#, Du X, Jiang H, Xie J*. Ndfip1 attenuated 6-OHDA-induced iron accumulation via regulating the degradation of DMT1. Neurobiol Aging. 2015 Feb; 36(2):1183-93.
2. Jiang H*, Wang J, Rogers J, Xie J*. Brain Iron Metabolism Dysfunction in Parkinson's Disease. Mol Neurobiol. 2017 May; 54(4):3078-3101.
3. Xu H*, Jiang H,Xie J*. New insights into the crosstalk between NMDARs and iron: implications for understanding pathology of neurological diseases. Front Mol Neurosci. 2017 Mar 16; 10: 71.
4. Yu X#, Du T#, Song N, He Q, Shen Y, Jiang H, Xie J*. Decreased iron levels in the temporal cortex in postmortem human brains with Parkinson’s disease. Neurology. 2013 Jan 29; 80(5):492-5.
5. Jiang H*, Song N, Xu H, Zhang S, Wang J, Xie J*. Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res. 2010 Mar; 20(3):345-56.
6. Du X#, Xu H#, Shi L, Jiang Z, Song N, Jiang H*, Xie J*. Activation of ATP-sensitive potassium channels enhances DMT1-mediated iron uptake in SK-N-SH cells in vitro. Sci Rep. 2016 Sep; 6:33674.
7. Song N, Wang J, Jiang H, Xie J*. Ferroportin1 but not hephaestin contributes to iron accumulation in a cell model of Parkinson’s disease. Free Radic Biol Med. 2010 Jan; 48(2): 332-41.
8. Wang J#, Bi M#, Liu H, Song N, Xie J*. The protective effect of lactoferrin on ventral mesencephalon neurons against MPP+is not connected with itsiron binding ability. Sci Rep. 2015 Jun 2; 5:10729.
9. Wang J*, Bi M, Xie J*. Ceruloplasmin is Involved in the Nigral Iron Accumulation of 6-OHDA-Lesioned Rats. Cell Mol Neurobiol. 2015 Jul; 35(5):661-8.
10. Zhang S#, Wang J#, Song N, Xie J*, Jiang H*. Up-regulation of divalent metal transporter 1 is involved in 1-methyl-4-phenylpyridinium (MPP+)-induced apoptosis in MES23.5 cells. Neurobiol Aging, 2009 Sep; 30(9):1466-76.
11. Liu K#, Xu H#, Xiang H, Sun P*, Xie J*. Protective effects of Ndfip1 on MPP+-induced apoptosis in MES23.5 cells and its underlying mechanisms.Exp Neurol. 2015 Aug; 273:215-224.
12. Li W, Jiang H, Song N, Xie J*. Dose-and time-dependent alpha-synuclein aggregation induced by ferric iron in SK-N-SH cells. Neurosci Bull. 2010 Jun; 26(3):205-10.
13. Lv Z, Jiang H, Xu H, Song N, Xie J*. Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson's disease. J Neural Transm. 2011 Mar; 118(3):361-9.
14. Li W, Jiang H, Song N, Xie J*. Oxidative Stress Partially Contributes to Iron-Induced Alpha-Synuclein Aggregation in SK-N-SH Cells. Neurotox Res. 2011 Apr; 19(3):435-42.
15. He Q, Song N, Jia F, Xu H, Yu X, Xie J, Jiang H*. Role of α-synuclein aggregation and the nuclear factor E2-related factor 2/heme oxygenase-1 pathway in iron-induced neurotoxicity. Int J Biochem Cell Biol. 2013 Jun; 45(6):1019-30.
16. Yu X#, Song N#, Guo X, Jiang H, Zhang H, Xie J*. Differences in vulnerability of neurons and astrocytes to heme oxygenase-1 modulation: Implications for mitochondrial ferritin. Sci Rep. 2016 Apr; 6:24200.
17. Zhang H, Wang N, Song N, Xu H, Shi L, Jiang H, Xie J*. 6-hydroxydopamine promotes iron traffic in primary cultured astrocytes. Biometals. 2013 Oct; 26(5):705-14.
18. Xu X#, Song N#, Wang R, Jiang H, Xie J*. Preferential Heme Oxygenase-1 Activation in Striatal Astrocytes Antagonizes Dopaminergic Neuron Degeneration in MPTP-Intoxicated Mice. Mol Neurobiol. 2016 Oct; 53(8):5056-65.
19. Wang J#, Song N#, Jiang H, Wang J, Xie J*. Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim Biophys Acta. 2013 May; 1832(5):618-25.
20. Zhang H#, Song N#, Jiang H, Bi M, Xie J*. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor inhibit ferrous iron influx via divalent metal transporter 1 and iron regulatory protein 1 regulation in ventral mesencephalic neurons. Biochim Biophys Acta. 2014 Dec; 1843(12):2967-75.
21. Jiao Q#, Du X#, Li Y, Gong B, Shi L, Tang T, Jiang H*. The neurological effects of ghrelin in brain diseases: Beyond metabolic functions. Neurosci Biobehav Rev. 2017 Feb; 73:98-111.
22. Shi L#, Du X#, Jiang H*, Xie J*. Ghrelin and Neurodegenerative Disorders-a Review. Mol Neurobiol. 2017 Mar; 54(2):1144-1155.
23. Shen X, Song N, Du X, Li Y, Xie J, Jiang H*. Nesfatin-1 protects dopaminergic neurons against MPP+/MPTP-induced neurotoxicity through the C-Raf-ERK1/2-dependent anti-apoptotic pathway. Sci Rep. 2017 Jan; 7:40961.
24. Shi L#, Bian X#, Qu Z, Ma Z, Zhou Y, Wang K*, Jiang H*,Xie J*. Peptide hormone ghrelin enhances neuronal excitability by inhibition of Kv7/KCNQ channels. Nat Commun. 2013 Feb; 4:1435.
25. Yu J#, Xu H#, Shen X, Jiang H*. Ghrelin protects MES23.5 cells against rotenone via inhibiting mitochondrial dysfunction and apoptosis. Neuropeptides. 2016 Apr; 56:69-74.
26. Zhao Z, Liu H, Xiao K, Yu M, Cui L, Zhu Q, Zhao R, Li G*, Zhou Y*. Ghrelin administration enhances neurogenesis but impairs spatial learning and memory in adult mice. Neuroscience. 2014 Jan 17; 257:175-85.
27. Du X, Xu H, Jiang H, Xie J*. Akt/Nrf2 activated upregulation of heme oxygenase-1 involves in the role of Rg1 against ferrous iron-induced neurotoxicity in SK-N-SH cells. Neurotox Res. 2013 Jul; 24(1):71-9.
28. Cui L#, Sun W#, Yu M, Li N, Guo L, Gu H, Zhou Y*. Disrupted-in-schizophrenia1 (DISC1) L100P mutation alters synaptic transmission and plasticity in the hippocampus and causes recognition memory deficits. Mol Brain. 2016 Oct 12; 9(1):89.
29. Xue Y#, Yang Y#, Liu H, Chen W, Chen A, Sheng Q, Chen X, Wang Y, Chen H, Liu H, Pang Y, Chen L*. Orexin-A increases the activity of globus pallidus neurons in both normal and parkinsonian rats. Eur J Neurosci. 2016 Sep; 44(5):2247-57.
30. Chen L#*, Xu R#, Sun F, Xue Y, Hao X, Liu H, Wang H, Chen X, Liu Z, Deng W, Han X, Xie J, Yung W.Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels regulate firing of globus pallidus neurons in vivo. Mol Cell Neurosci. 2015 Sep; 68:46-55.
# These authors are the co-first authors; *Corresponding author
Thank you for your attention.
Sincerely,
Junxia Xie
Professor and Dean
Institute of Brain Science and Disease
Qingdao University
China
Address: 308 Ningxia Road, Qingdao, 266071, P.R. China
Tel: 086-0532-83780035 ;Fax: 086-0532-83780136;Email: ibsd@qdu.edu.cn